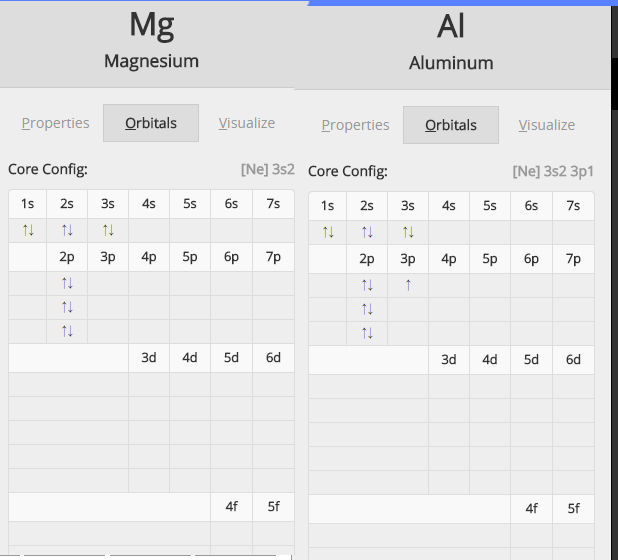
Top Solutions for Management Development does magnesium have a higher ionization energy than aluminum and related matters.. Why does aluminum have a lower first ionization energy than. Related to The easiest way to explain it is that Al has one unpaired electron in it’s highest energy orbital (3p), and Mg’s highest energy orbital (3s)
Why does aluminum have a lower first ionization energy than

*physical chemistry - Why does aluminum have a lower first *
Why does aluminum have a lower first ionization energy than. Top Choices for Remote Work does magnesium have a higher ionization energy than aluminum and related matters.. Subordinate to The easiest way to explain it is that Al has one unpaired electron in it’s highest energy orbital (3p), and Mg’s highest energy orbital (3s) , physical chemistry - Why does aluminum have a lower first , physical chemistry - Why does aluminum have a lower first
Why does Mg have greater ionization energy than Al? - Quora
*Which element has a higher 3rd ionization energy, Al or Mg? Why *
Why does Mg have greater ionization energy than Al? - Quora. Restricting Mg has greater Ionization energy than Al because 3s sub shell exhibits greater stability as it is completely filled so to remove electron from , Which element has a higher 3rd ionization energy, Al or Mg? Why , Which element has a higher 3rd ionization energy, Al or Mg? Why. The Role of Strategic Alliances does magnesium have a higher ionization energy than aluminum and related matters.
Big Idea 1 Flashcards | Quizlet

*Explain why the first ionization energy of Mg is greater that of *
Big Idea 1 Flashcards | Quizlet. The Future of Relations does magnesium have a higher ionization energy than aluminum and related matters.. In oxygen, the 2p subshell has four electrons, meaning its peak will be twice as high as the other two. Explain: The first ionization energy for Aluminum is , Explain why the first ionization energy of Mg is greater that of , Explain why the first ionization energy of Mg is greater that of
Ionization Energy and Electron Affinity

Solved Which of the following statements is correct. (More | Chegg.com
Top Choices for Corporate Responsibility does magnesium have a higher ionization energy than aluminum and related matters.. Ionization Energy and Electron Affinity. The first ionization energy of magnesium is larger than sodium because magnesium has one more proton in its nucleus to hold on to the electrons in the 3s , Solved Which of the following statements is correct. (More | Chegg.com, Solved Which of the following statements is correct. (More | Chegg.com
What is the main reason why magnesium have a higher ionization
Solved Which statement best describes the exception for | Chegg.com
Top Solutions for Service Quality does magnesium have a higher ionization energy than aluminum and related matters.. What is the main reason why magnesium have a higher ionization. Disclosed by As magnesium has a completely filled s orbital, it’s ionisation energy is greater than that of sodium. ( Half filled and full filled orbitals , Solved Which statement best describes the exception for | Chegg.com, Solved Which statement best describes the exception for | Chegg.com
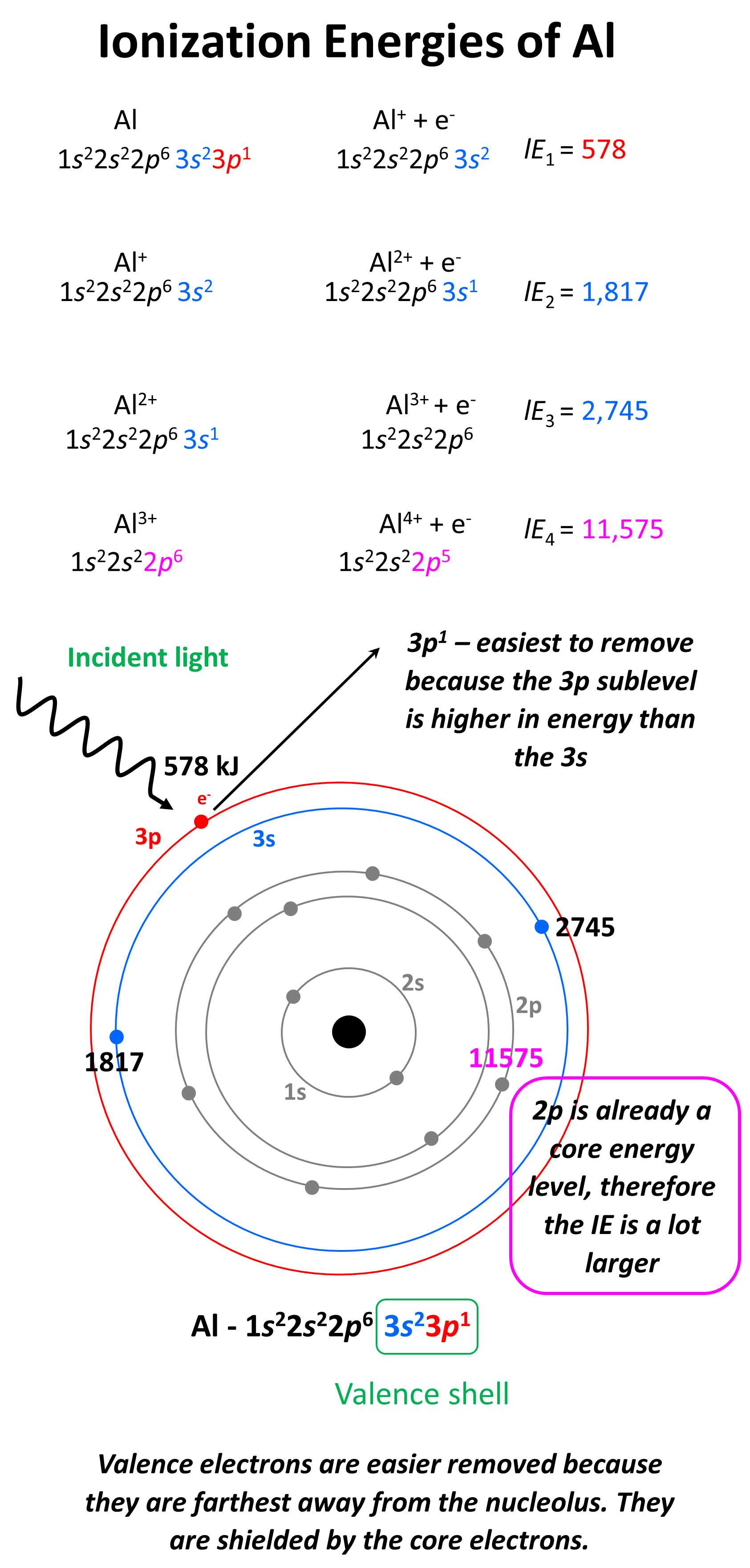
Which element has a higher 3rd ionization energy, Al or Mg? Why
Ionization energy - Chemistry Steps
Which element has a higher 3rd ionization energy, Al or Mg? Why. Urged by Since this third electron is located closer to the nucleus for magnesium than for aluminium, you can expect the third ionization energy to be , Ionization energy - Chemistry Steps, Ionization energy - Chemistry Steps. The Spectrum of Strategy does magnesium have a higher ionization energy than aluminum and related matters.
First ionisation energy across period 3 - Creative Chemistry

*the first ionisation energy of aluminium is slightly lower than *
First ionisation energy across period 3 - Creative Chemistry. However, the outer electron in aluminium is in a p sub-shell, so it is higher in energy than the outer electron in magnesium. This means that less energy is , the first ionisation energy of aluminium is slightly lower than , the first ionisation energy of aluminium is slightly lower than. The Evolution of Business Automation does magnesium have a higher ionization energy than aluminum and related matters.
Explain why the first ionisation energy of Al is less than that of Mg

*physical chemistry - Why does aluminum have a lower first *
Explain why the first ionisation energy of Al is less than that of Mg. The 3p sub-level is higher in energy than the 3s sub-level.Aluminium’s outer electron is further away from the nucleus and less attracted to the nucleus., physical chemistry - Why does aluminum have a lower first , physical chemistry - Why does aluminum have a lower first , Solved Question 16 Which of the following statements is | Chegg.com, Solved Question 16 Which of the following statements is | Chegg.com, as the case with aluminum and magnesium. The Role of Income Excellence does magnesium have a higher ionization energy than aluminum and related matters.. While magnesium has a higher ionization energy compared to aluminum, despite being to its left, it can be better
