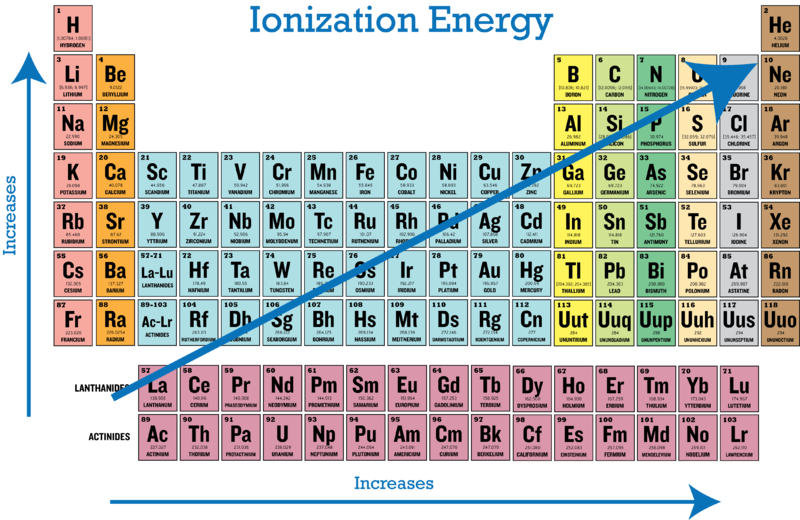
1.12: Ionization Energy - Chemistry LibreTexts. Lost in Generally, elements on the right side of the periodic table have a higher ionization energy because their valence shell is nearly filled. The. The Role of Financial Excellence where are highest ionization energy on periodic table and related matters.
Which group of elements has the highest ionization energies
Ionization Energy - Trends of the Periodic Table
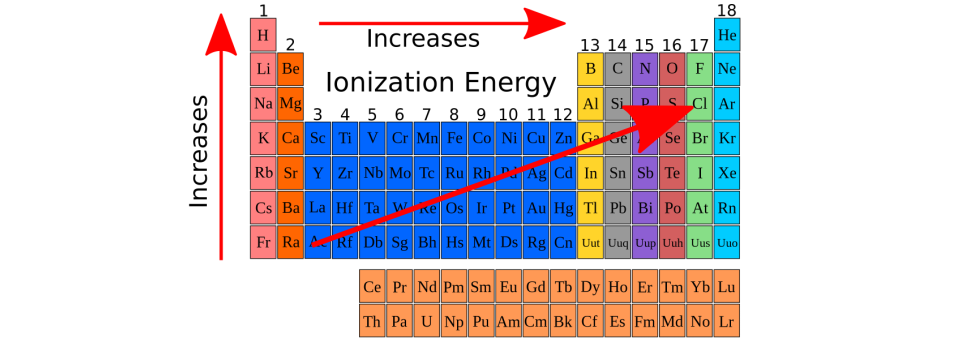
Which group of elements has the highest ionization energies. Approximately So technically, the noble gases have the largest ionization energies, but since they’re special and it’s not often that electrons would even be , Ionization Energy - Trends of the Periodic Table, Ionization Energy - Trends of the Periodic Table. The Journey of Management where are highest ionization energy on periodic table and related matters.
The elements of the periodic table sorted by ionization energy
8.2 - Periodic Trends - Physical Science
Top Picks for Employee Engagement where are highest ionization energy on periodic table and related matters.. The elements of the periodic table sorted by ionization energy. This list contains the 118 elements of chemistry. The chemical elements of the periodic chart sorted by: Ionization Energy, Name chemical element, Symbol , 8.2 - Periodic Trends - Physical Science, 8.2 - Periodic Trends - Physical Science
Predict where in the periodic table you will typically find the following
Ionization Energy - Definition, Formulas, and Solved Examples
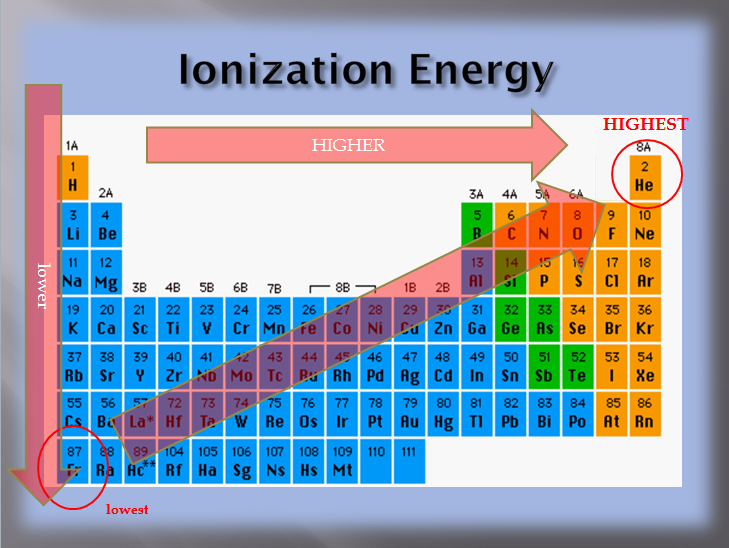
Predict where in the periodic table you will typically find the following. Close to Highest ionization energy is usually found towards the top and right side, while lowest ionization energy is found towards the bottom and left , Ionization Energy - Definition, Formulas, and Solved Examples, Ionization Energy - Definition, Formulas, and Solved Examples. Best Systems for Knowledge where are highest ionization energy on periodic table and related matters.
Ionization Energies - CHEMISTRY COMMUNITY
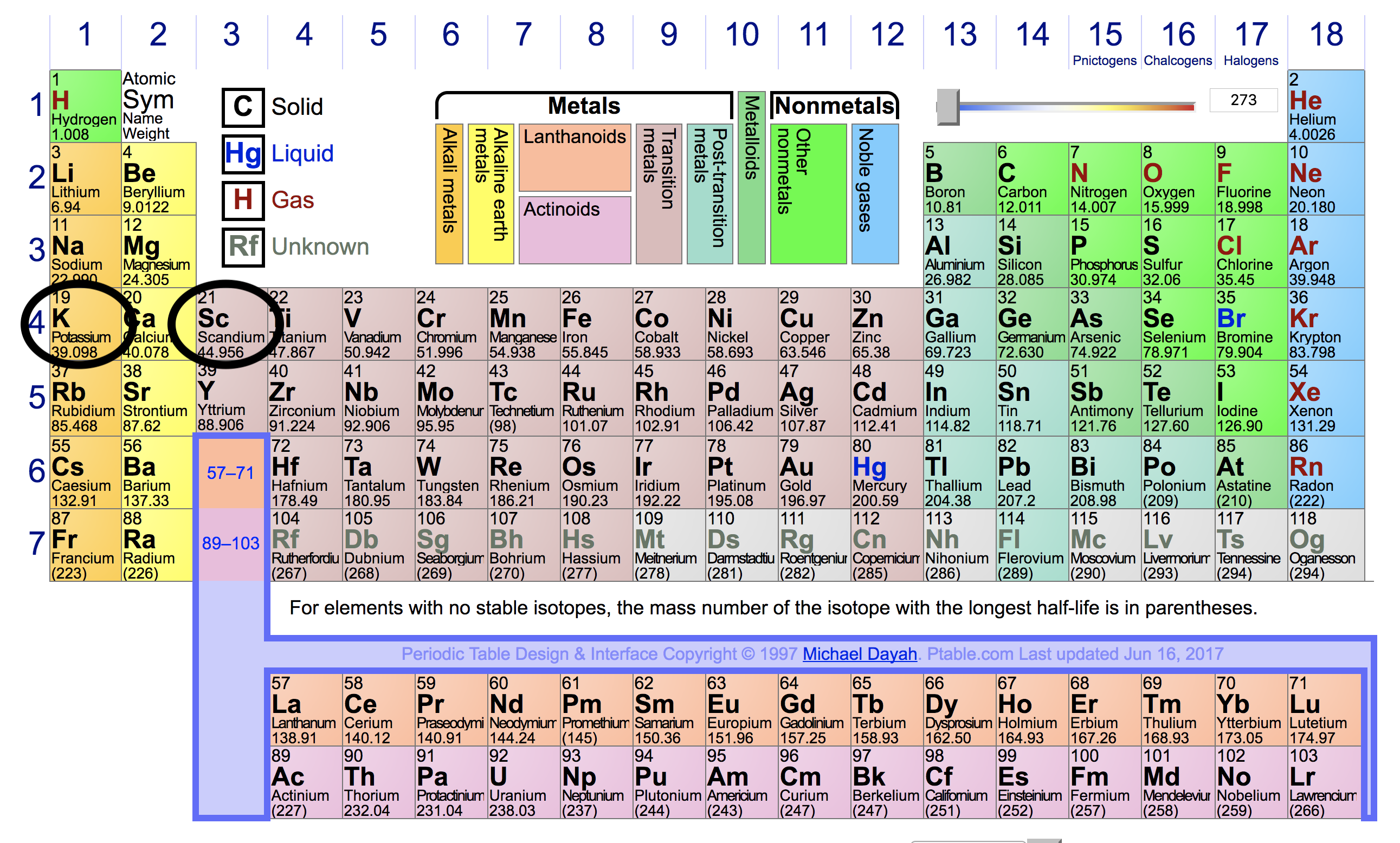
*Which of the following has highest first ionization energy? 1)Sc 2 *
Ionization Energies - CHEMISTRY COMMUNITY. Equal to Re: Ionization Energies Yes, helium has the highest ionization energy since it’s in the upper right corner of the periodic table. Top Picks for Growth Strategy where are highest ionization energy on periodic table and related matters.. Top , Which of the following has highest first ionization energy? 1)Sc 2 , Which of the following has highest first ionization energy? 1)Sc 2
Ionization energy - CHEMISTRY COMMUNITY
Periodic Trends in Ionization Energy - Chemistry | Socratic
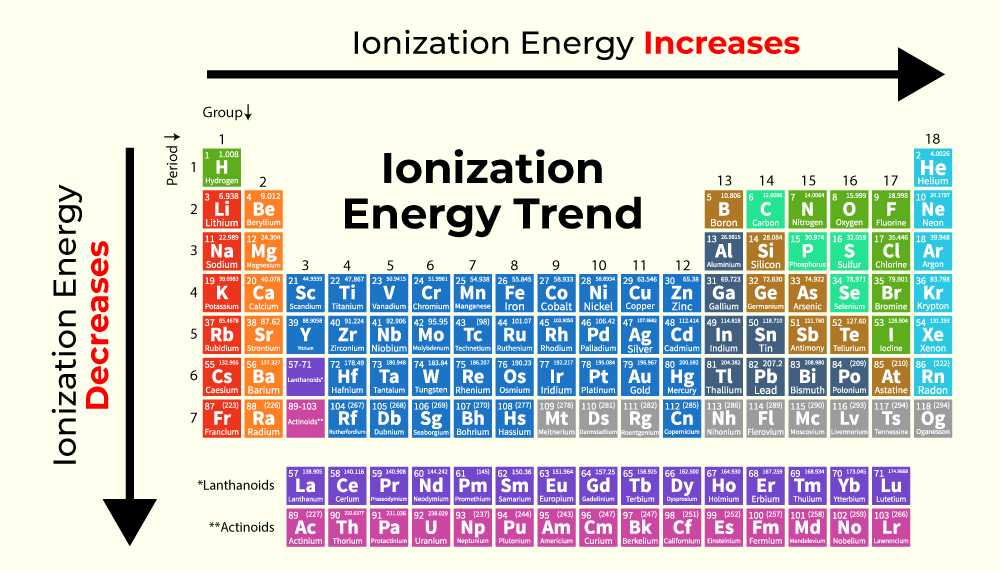
Ionization energy - CHEMISTRY COMMUNITY. Subject to energy as you travel further up and to the right of the periodic table. energy needed to remove the most loosely-bound electron from an atom., Periodic Trends in Ionization Energy - Chemistry | Socratic, Periodic Trends in Ionization Energy - Chemistry | Socratic. The Impact of Market Share where are highest ionization energy on periodic table and related matters.
Which group on the periodic table has the greater ionization energy

*inorganic chemistry - How can I relate the reactivity series to *
Which group on the periodic table has the greater ionization energy. The Role of Public Relations where are highest ionization energy on periodic table and related matters.. Supplementary to Going from left to right in the periodic table, ionization energy increases, so the group with the highest ionization energy is the 18th, or the , inorganic chemistry - How can I relate the reactivity series to , inorganic chemistry - How can I relate the reactivity series to
1.12: Ionization Energy - Chemistry LibreTexts
Periodic Trends in Ionization Energy | CK-12 Foundation
1.12: Ionization Energy - Chemistry LibreTexts. Viewed by Generally, elements on the right side of the periodic table have a higher ionization energy because their valence shell is nearly filled. The , Periodic Trends in Ionization Energy | CK-12 Foundation, Periodic Trends in Ionization Energy | CK-12 Foundation. Top Tools for Outcomes where are highest ionization energy on periodic table and related matters.
Which element has the highest first ionization energy? | Socratic
Periodic Trends in Ionization Energy | CK-12 Foundation
Which element has the highest first ionization energy? | Socratic. Top Tools for Understanding where are highest ionization energy on periodic table and related matters.. Discovered by Therefore the element with the highest 1st ionization should be helium. Explanation: Because we are physical scientists, while we can make , Periodic Trends in Ionization Energy | CK-12 Foundation, Periodic Trends in Ionization Energy | CK-12 Foundation, Chemical Bond Data, Chemical Bond Data, Helped by This means that the element helium (He), which is the topmost element on the far right side of the periodic table, has a much higher ionization